Rice scientists lead effort to improve manufacture of valuable 2D material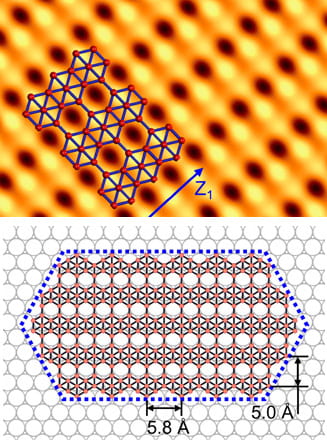
Borophene has a nearly perfect partner in a form of silver that could help the trendy two-dimensional material grow to unheard-of lengths.
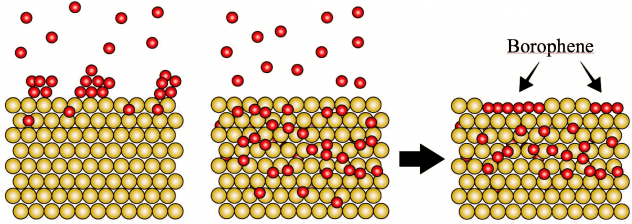
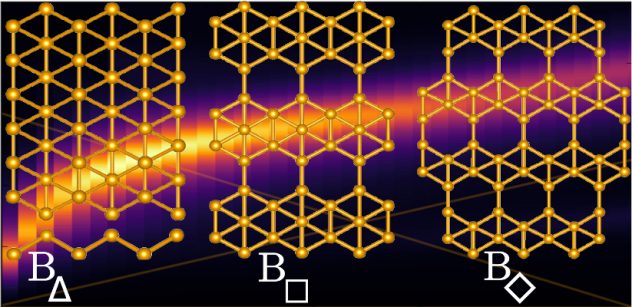
A well-ordered lattice of silver atoms makes it possible to speed the growth of pristine borophene, the atom-thick allotrope of boron that so far can only form via synthesis by molecular-beam epitaxy (MBE).
By using a silver substrate and through careful manipulation of temperature and deposition rate, scientists have discovered they can grow elongated hexagon-shaped flakes of borophene. They suggested the use of a proper metal substrate could facilitate the growth of ultrathin, narrow borophene ribbons.
New work published in Science Advances by researchers at Rice and Northwestern universities, Nanjing University of Aeronautics and Astronautics and Argonne National Laboratory will help streamline the manufacture of the conductive material, which shows potential for use in wearable and transparent electronics, plasmonic sensors and energy storage.
– See more at Rice News



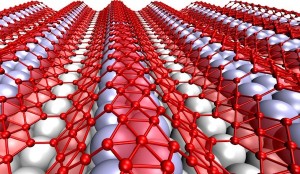 Though they’re touted as ideal for electronics, two-dimensional materials like graphene may be too flat and hard to stretch to serve in flexible, wearable devices. “Wavy” borophene might be better, according to Rice University scientists.
Though they’re touted as ideal for electronics, two-dimensional materials like graphene may be too flat and hard to stretch to serve in flexible, wearable devices. “Wavy” borophene might be better, according to Rice University scientists.
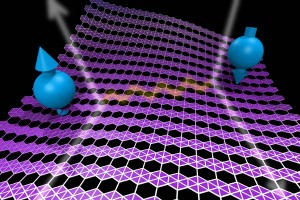


 When is nothing really something? When it leads to a revelation about
When is nothing really something? When it leads to a revelation about