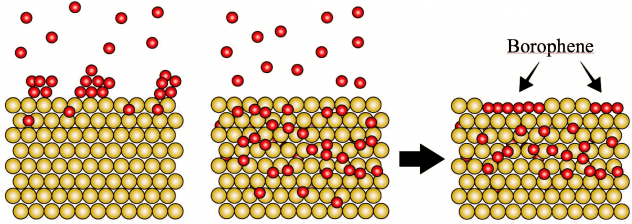
Simulations could help molecular engineers enhance creation of semiconducting nanomaterials
 Scientific studies describing the most basic processes often have the greatest impact in the long run. A new work by Rice University engineers could be one such, and it’s a gas, gas, gas for nanomaterials.
Scientific studies describing the most basic processes often have the greatest impact in the long run. A new work by Rice University engineers could be one such, and it’s a gas, gas, gas for nanomaterials.
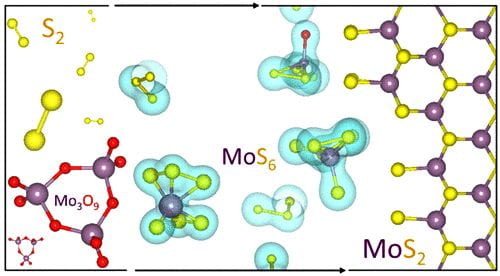
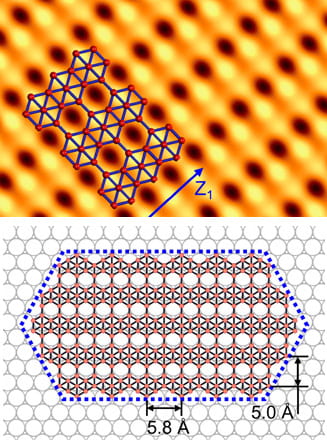
Rice materials theorist Boris Yakobson, graduate student Jincheng Lei and alumnus Yu Xie of Rice’s Brown School of Engineering have unveiled how a popular 2D material, molybdenum disulfide (MoS2), flashes into existence during chemical vapor deposition (CVD).
Knowing how the process works will give scientists and engineers a way to optimize the bulk manufacture of MoS2 and other valuable materials classed as transition metal dichalcogenides (TMDs), semiconducting crystals that are good bets to find a home in next-generation electronics.
Their study in the American Chemical Society journal ACS Nano focuses on MoS2‘s “pre-history”, specifically what happens in a CVD furnace once all the solid ingredients are in place. CVD, often associated with graphene and carbon nanotubes, has been exploited to make a variety of 2D materials by providing solid precursors and catalysts that sublimate into gas and react. The chemistry dictates which molecules fall out of the gas and settle on a substrate, like copper or silicone, and assemble into a 2D crystal.
– See more at Rice News

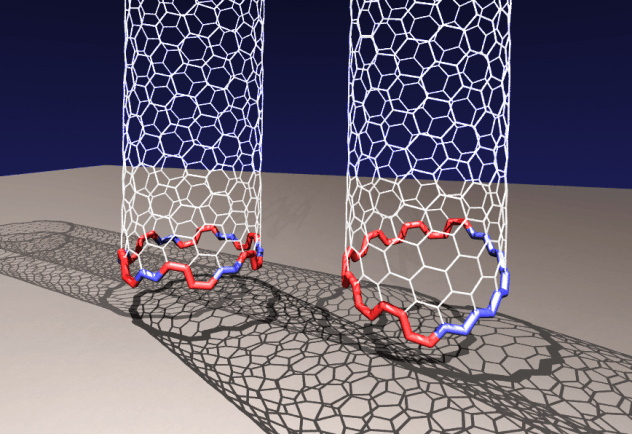 When is a circle less stable than a jagged loop? Apparently when you’re talking about carbon nanotubes.
When is a circle less stable than a jagged loop? Apparently when you’re talking about carbon nanotubes.