Rice model may lead to better materials for aerospace, automotive, medical applications
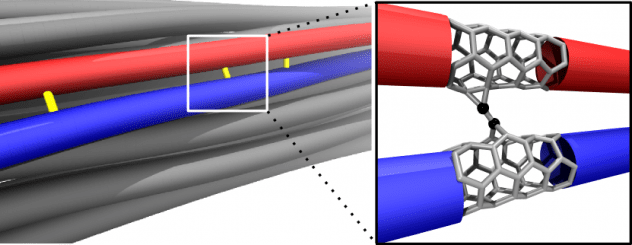 Carbon nanotube fibers are not nearly as strong as the nanotubes they contain, but Rice University researchers are working to close the gap.
Carbon nanotube fibers are not nearly as strong as the nanotubes they contain, but Rice University researchers are working to close the gap.
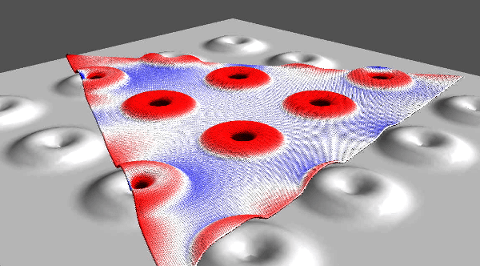
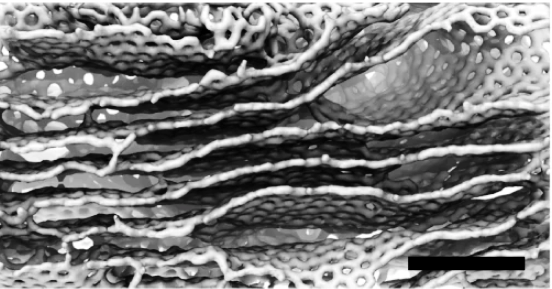
A computational model by materials theorist Boris Yakobson and his team at Rice’s Brown School of Engineering establishes a universal scaling relationship between nanotube length and friction between them in a bundle, parameters that can be used to fine-tune fiber properties for strength.
The model is a tool for scientists and engineers who develop conductive fibers for aerospace, automotive, medical and textile applications like smart clothing. Carbon nanotube fibers have been considered as a possible basis for a space elevator, a project Yakobson has studied.
The research is detailed in the American Chemical Society journal ACS Nano.
– See more at Rice News



 Sunny Gupta, a fourth-year graduate student in Yakobson’s Group, has received a “Best Oral Presentation” Award at the
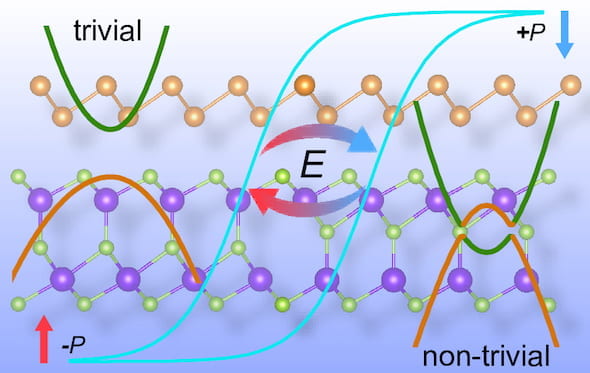
Sunny Gupta, a fourth-year graduate student in Yakobson’s Group, has received a “Best Oral Presentation” Award at the 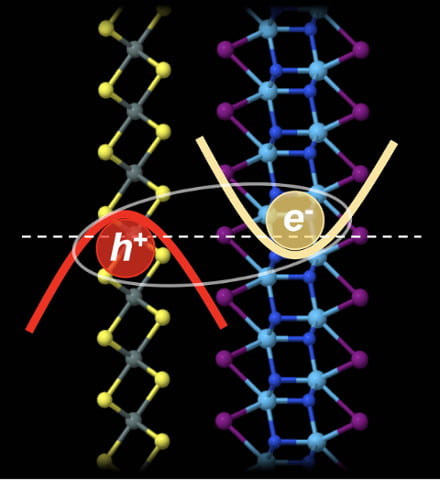 Mixing and matching computational models of 2D materials led scientists at Rice University to the realization that
Mixing and matching computational models of 2D materials led scientists at Rice University to the realization that 
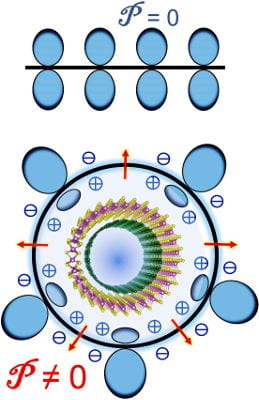 One nanotube could be great for electronics applications, but there’s new evidence that two could be tops.
One nanotube could be great for electronics applications, but there’s new evidence that two could be tops.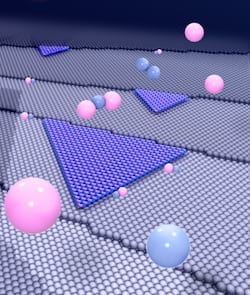 Step by step, scientists are figuring out new ways to extend
Step by step, scientists are figuring out new ways to extend 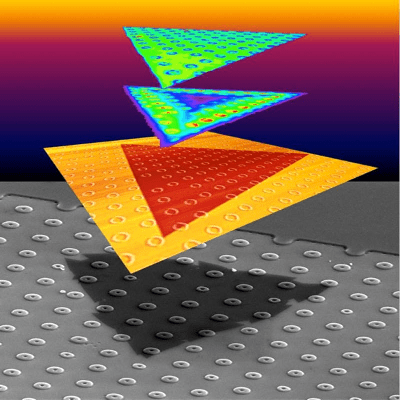
 bulk quantities of just about any carbon source into valuable graphene flakes. The process is quick and cheap; Tour said the “flash graphene” technique can convert a ton of coal, food waste or plastic into graphene for a fraction of the cost used by other bulk graphene-producing methods.
bulk quantities of just about any carbon source into valuable graphene flakes. The process is quick and cheap; Tour said the “flash graphene” technique can convert a ton of coal, food waste or plastic into graphene for a fraction of the cost used by other bulk graphene-producing methods. Boris I. Yakobson, the Karl F. Hasselmann Chair of Engineering, Professor of Materials Science & NanoEngineering and of Chemistry, has been named a
Boris I. Yakobson, the Karl F. Hasselmann Chair of Engineering, Professor of Materials Science & NanoEngineering and of Chemistry, has been named a  The Fall 2019 issue of the
The Fall 2019 issue of the 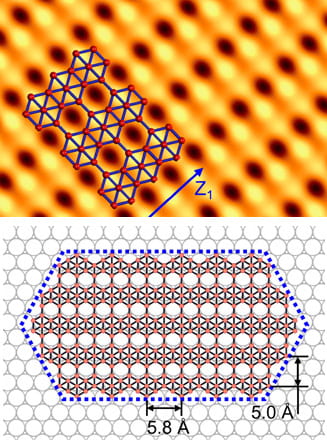
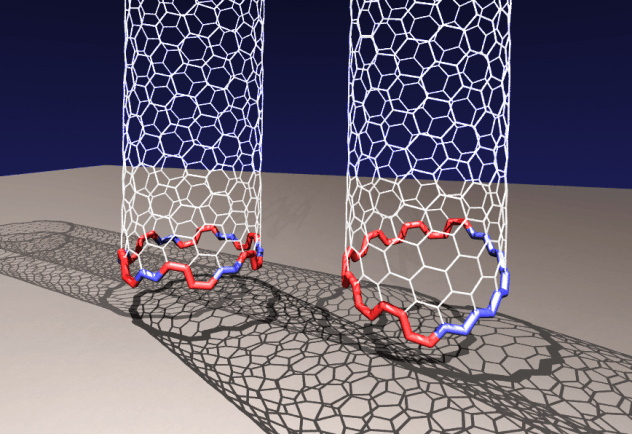 When is a circle less stable than a jagged loop? Apparently when you’re talking about carbon nanotubes.
When is a circle less stable than a jagged loop? Apparently when you’re talking about carbon nanotubes.