Rice, Argonne, Northwestern scientists show unique mechanism makes valuable 2D material
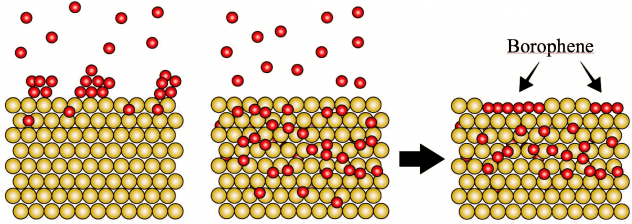
In the heat of a furnace, boron atoms happily dive into a bath of gold. And when things get cool, they resurface as coveted borophene.
The discovery by scientists from Rice University, Argonne National Laboratory and Northwestern University is a step toward practical applications like wearable or transparent electronics, plasmonic sensors or energy storage for the two-dimensional material with excellent conductivity.
Teams led by Boris Yakobson at Rice, Nathan Guisinger at Argonne and Mark Hersam at Northwestern both formed the theory for and then demonstrated their novel method to grow borophene – the atom-thick form of boron – on a gold surface.
They found that with sufficient heat in a high vacuum, boron atoms streamed into the furnace sink into the gold itself. Upon cooling, the boron atoms reappear and form islands of borophene on the surface.
This is distinct from most other 2D materials made by feeding gases into a furnace. In standard chemical vapor deposition, the atoms settle onto a substrate and connect with each other. They typically don’t disappear into the substrate.
The discovery was described in a paper in the American Chemical Society journal ACS Nano.
– See more at Rice News