Rice University scientists find new workable feature of a class of 2D materials
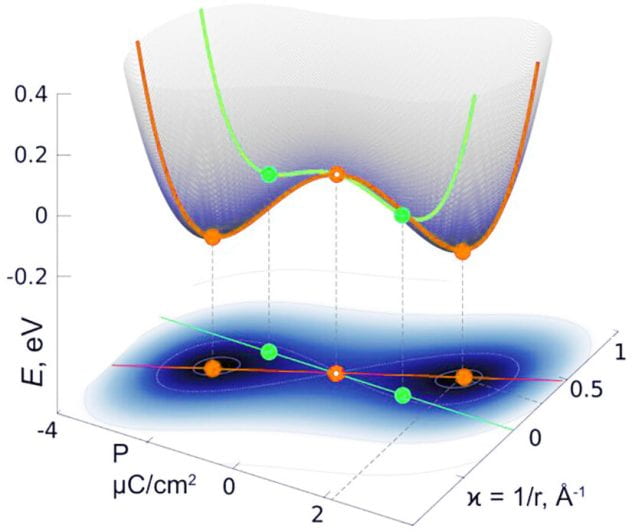 Rice University materials scientist Boris Yakobson and collaborators uncovered a property of ferroelectric 2D materials that could be exploited as a feature in future devices.
Rice University materials scientist Boris Yakobson and collaborators uncovered a property of ferroelectric 2D materials that could be exploited as a feature in future devices.
Because they bend in response to an electrical stimulus, single-layer ferroelectric materials can be controlled to act as a nanoscale switch or even a motor, according to the study published in ACS Nano.
Polarization drives the larger atoms to one side of the 2D-material layer and the smaller atoms to the other side. This asymmetrical distribution of the atoms or ions causes the material surface to bend in ferroelectric state.
The study looked at 2D indium phosphide (InP) as a representative of the class of ferroelectrics for which it predicts this property.
“This new property or flexing behavior has to be tested in a laboratory for specific substances,” Yakobson said. “Its most likely use will be as a type of switch. This behavior is very fast, very sensitive, which means that with a very tiny local signal you can maybe switch on a turbine or electrical engine, or control adaptive-optics telescopes’ mirrors. That’s basically the essence of these actuators.”
– See more at Rice News



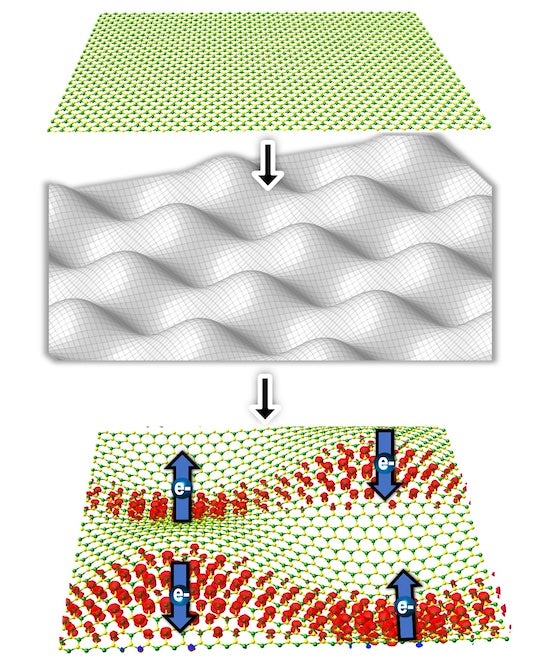 contoured substrates
contoured substrates
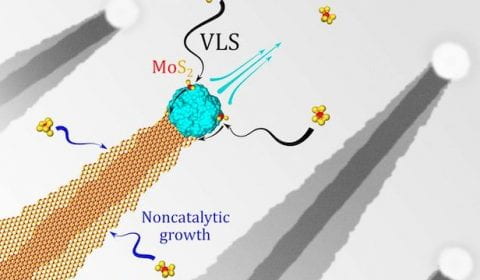 It’s now possible to quickly make ultrathin nanoribbons of
It’s now possible to quickly make ultrathin nanoribbons of 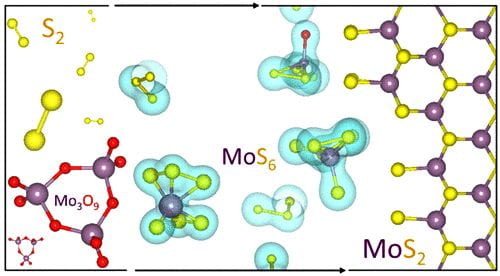 Scientific studies describing the most basic processes often have the greatest impact in the long run. A new work by Rice University engineers could be one such, and it’s a gas, gas, gas for nanomaterials.
Scientific studies describing the most basic processes often have the greatest impact in the long run. A new work by Rice University engineers could be one such, and it’s a gas, gas, gas for nanomaterials.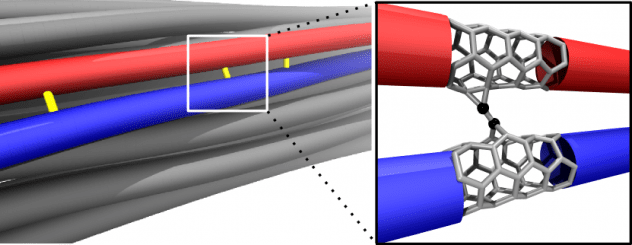 Carbon nanotube fibers are not nearly as strong as the nanotubes they contain, but Rice University researchers are working to close the gap.
Carbon nanotube fibers are not nearly as strong as the nanotubes they contain, but Rice University researchers are working to close the gap.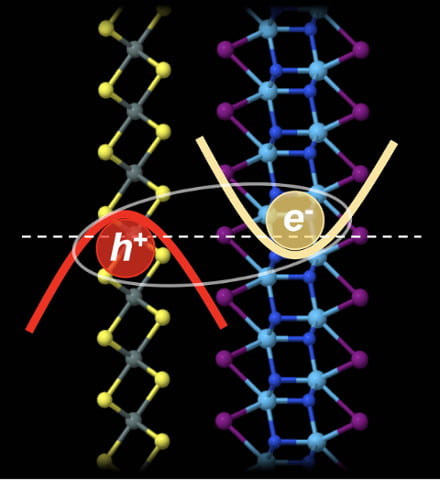 Mixing and matching computational models of 2D materials led scientists at Rice University to the realization that
Mixing and matching computational models of 2D materials led scientists at Rice University to the realization that 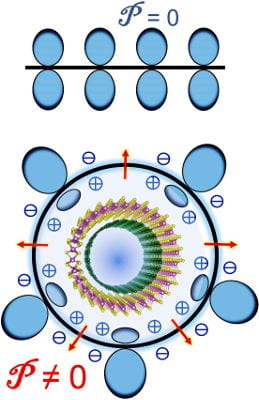 One nanotube could be great for electronics applications, but there’s new evidence that two could be tops.
One nanotube could be great for electronics applications, but there’s new evidence that two could be tops. bulk quantities of just about any carbon source into valuable graphene flakes. The process is quick and cheap; Tour said the “flash graphene” technique can convert a ton of coal, food waste or plastic into graphene for a fraction of the cost used by other bulk graphene-producing methods.
bulk quantities of just about any carbon source into valuable graphene flakes. The process is quick and cheap; Tour said the “flash graphene” technique can convert a ton of coal, food waste or plastic into graphene for a fraction of the cost used by other bulk graphene-producing methods.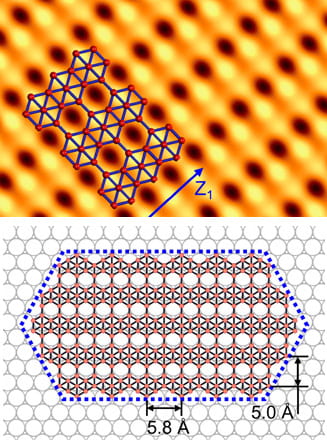
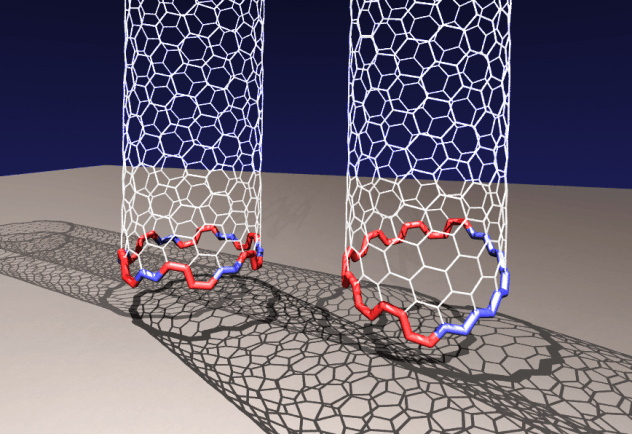 When is a circle less stable than a jagged loop? Apparently when you’re talking about carbon nanotubes.
When is a circle less stable than a jagged loop? Apparently when you’re talking about carbon nanotubes.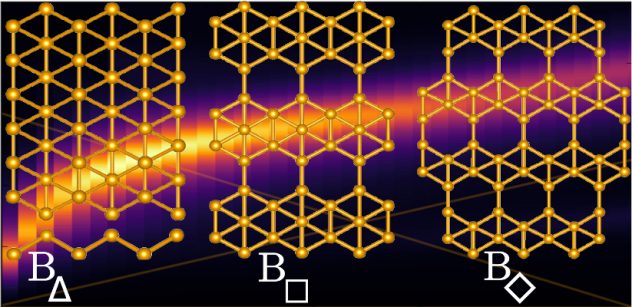
 Rice scientists calculate tweaks to graphene would form phonon-friendly cones
Rice scientists calculate tweaks to graphene would form phonon-friendly cones